What is Research?
The aim of clinical research is to gather evidence that can be applied for improved understanding of health and healthcare, and the development of safe and effective treatments.
Most clinical research in the NHS will take the form of a research study or a clinical trial. A research study aims to better understand health and health conditions and may gather information from a person about their history, medical condition, treatments and outcomes. A research study collates information but does not introduce new or experimental treatment. Experimental research usually takes the form of a clinical trial. A clinical trial compares the effects of two treatments, usually either a new drug (but can include new technologies or methods), or the use of an existing drug in a medical condition that it has not previously been used for.
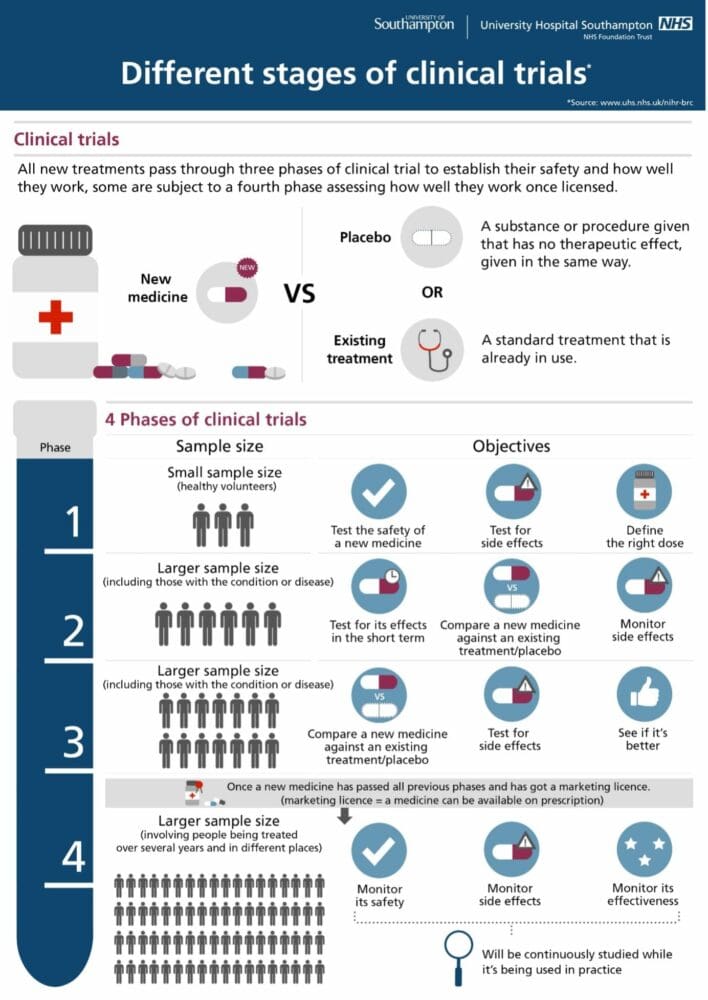
Infographic courtesy of Southampton National Institute for Health Research Clinical Research Facility
Phases of Clinical Trials
Pre-clinical studies gather information about feasibility and safety in a laboratory setting before any testing in humans can begin.

Research Results and Clinical Practice
Research projects vary in size and duration, and historically, it can take many years to bring new products to market. To address this delay, efforts have been made to shorten the time required to translate research results into clinical practice, a process known as “from bench to bedside.”
However, clinical trials still require time to gather all necessary information to determine the trial’s success and make new treatments available to the general population. For a new treatment or medication to be approved for use in the NHS, it must receive approval from the National Institute for Health and Care Excellence (NICE).